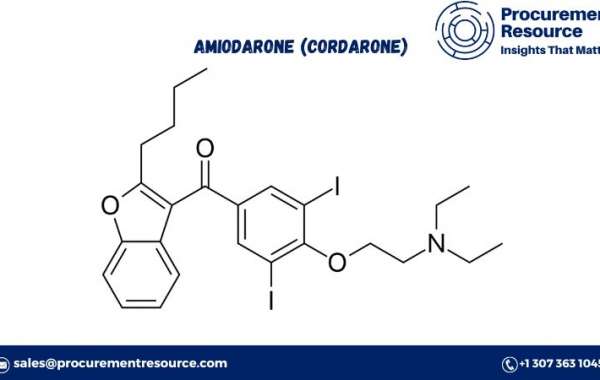
Amiodarone, commonly known by its brand name Cordarone, is a potent antiarrhythmic medication widely used to treat and prevent various types of cardiac arrhythmias. Understanding the Amiodarone (Cordarone) Production Process with Cost Analysis is crucial for pharmaceutical manufacturers and stakeholders in the healthcare sector. This comprehensive report delves into the production process of Amiodarone, covering procurement resource assessment, an overview of the drug, market drivers, raw material requirements, and a detailed breakdown of costs and key process information. Additionally, it offers insights into how a personalized report can substantiate your business strategies.
Request Free Sample - https://www.procurementresource.com/production-cost-report-store/amiodarone/request-sample
Procurement Resource Assessment: Amiodarone (Cordarone) Production Process
The Procurement Resource Assessment for Amiodarone production involves evaluating and securing the necessary resources required for efficient and cost-effective production. This assessment includes sourcing high-quality raw materials, establishing a reliable supply chain, setting up the production facility, and ensuring regulatory compliance.
- Raw Material Sourcing: The production of Amiodarone involves the synthesis of complex organic molecules, requiring high-purity raw materials and intermediates. Securing a stable and cost-effective supply of these raw materials is critical for maintaining consistent production and controlling costs. Establishing long-term contracts with reliable chemical suppliers, or in some cases, developing in-house synthesis capabilities, is essential for ensuring the quality and availability of these critical inputs.
- Plant Setup and Infrastructure: Setting up a production facility for Amiodarone requires significant investment in specialized equipment, including reactors, filtration units, crystallizers, and drying systems. The procurement of high-quality equipment and advanced technology is crucial to ensure the plant’s operational efficiency, product quality, and safety. Modern facilities must also be designed to meet stringent regulatory standards for pharmaceutical manufacturing, including Good Manufacturing Practices (GMP).
- Energy Supply: The production of Amiodarone is energy-intensive, particularly during the synthesis, purification, and drying stages. Securing a reliable and cost-effective energy supply, whether from electricity, steam, or alternative sources, is crucial for optimizing production costs and ensuring the sustainability of operations. Implementing energy-efficient technologies can help reduce operational costs and environmental impact.
- Labor and Expertise: Skilled labor and technical expertise are required to operate and maintain an Amiodarone production facility. The procurement of qualified personnel, including organic chemists, process engineers, and quality control experts, is essential for the successful operation of the plant. Continuous training and development programs ensure that staff remain updated with the latest industry practices and technological advancements.
- Regulatory Compliance: Compliance with Good Manufacturing Practices (GMP) and other regulatory standards is critical in the production of Amiodarone, a pharmaceutical product. Procuring the necessary systems and processes to meet these regulations is essential for avoiding penalties and ensuring the safety and efficacy of the product. This includes establishing robust quality control systems, documentation, and validation procedures.
Understanding Amiodarone (Cordarone)
Amiodarone (Cordarone) is a widely used antiarrhythmic medication that is effective in treating various types of cardiac arrhythmias, including atrial fibrillation, ventricular tachycardia, and ventricular fibrillation. Amiodarone works by prolonging the duration of the cardiac action potential and refractory period, thereby stabilizing the heart's rhythm.
Amiodarone is known for its complex pharmacokinetics, including a long half-life and extensive tissue distribution. Due to its potent effects and potential side effects, it is typically used in cases where other antiarrhythmic drugs have failed or are contraindicated.
The production of Amiodarone involves multiple steps, including the synthesis of the active pharmaceutical ingredient (API), followed by purification, crystallization, and formulation into the final dosage form, such as tablets or injectable solutions. Given its critical role in managing life-threatening arrhythmias, the production process of Amiodarone is highly regulated to ensure the safety, purity, and efficacy of the final product.
Market Drivers
The Market Drivers for Amiodarone production are influenced by several factors, including the prevalence of cardiac arrhythmias, advancements in pharmaceutical technology, regulatory approvals, and healthcare policies.
- Prevalence of Cardiac Arrhythmias: The global prevalence of cardiac arrhythmias, particularly atrial fibrillation and ventricular tachycardia, is on the rise, driven by factors such as aging populations, increasing rates of hypertension, and lifestyle changes. This growing prevalence has led to an increased demand for effective antiarrhythmic medications like Amiodarone, driving the market for its production.
- Advancements in Pharmaceutical Technology: Technological advancements in pharmaceutical manufacturing, including improvements in synthetic chemistry, purification techniques, and formulation processes, have enhanced the production efficiency and quality of Amiodarone. These innovations have enabled manufacturers to reduce production costs, increase yields, and meet the growing demand for high-quality antiarrhythmic drugs.
- Regulatory Approvals and Healthcare Policies: The production and distribution of Amiodarone are heavily influenced by regulatory approvals and healthcare policies. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), play a critical role in approving the use of Amiodarone for various indications, impacting its market availability. Additionally, healthcare policies that prioritize the treatment of cardiovascular diseases have further driven the demand for Amiodarone.
- Rising Healthcare Expenditure: The increasing healthcare expenditure in both developed and emerging markets has led to greater access to advanced medical treatments, including the use of Amiodarone for managing cardiac arrhythmias. As healthcare systems continue to invest in the treatment of cardiovascular diseases, the demand for effective medications like Amiodarone is expected to grow.
Raw Materials Requirements
The Raw Materials Requirements for Amiodarone production include essential inputs such as high-purity chemical precursors, solvents, and reagents used in the synthesis of the active pharmaceutical ingredient (API).
- Chemical Precursors: The synthesis of Amiodarone requires specific chemical precursors, including 2-butylbenzofuran and diethylamine, among others. These precursors are critical for the formation of the core structure of Amiodarone and must be of high purity to ensure the quality and efficacy of the final product. The procurement of these chemicals involves sourcing from reputable suppliers and ensuring consistency in their quality.
- Solvents and Reagents: Various solvents and reagents are used in the multi-step synthesis of Amiodarone. These include organic solvents such as methanol, ethanol, and acetone, as well as reagents like hydrochloric acid and sodium hydroxide. The selection and procurement of these materials are crucial for optimizing the reaction conditions and ensuring high yields of the API.
- Catalysts and Additives: Catalysts and additives are often used to enhance the efficiency of the chemical reactions involved in the production of Amiodarone. These may include transition metal catalysts or other specialized reagents that facilitate specific steps in the synthesis. The procurement of these materials requires careful consideration of their quality, availability, and cost.
- Water and Energy: High-purity water is essential in various stages of Amiodarone production, including synthesis, purification, and formulation. Energy is also a significant requirement, particularly for maintaining the necessary reaction conditions and during the drying and crystallization processes. Ensuring the availability of high-quality water and a reliable energy supply is critical for maintaining the efficiency and consistency of the production process.
Costs and Key Process Information
The Costs and Key Process Information for Amiodarone production involve the expenses associated with raw material procurement, energy consumption, labor, plant maintenance, and regulatory compliance. Understanding these costs is essential for managing the profitability and efficiency of Amiodarone production.
- Raw Material Costs: The cost of chemical precursors, solvents, and reagents is a primary expense in Amiodarone production. Fluctuations in the prices of these raw materials can significantly impact the overall production cost. Securing long-term supply contracts or exploring alternative raw material sources can help mitigate price volatility and ensure consistent production.
- Energy Costs: Energy consumption is a major component of the Amiodarone production process. The costs associated with maintaining the necessary reaction conditions, as well as the purification, drying, and crystallization processes, must be carefully managed to optimize production efficiency. Advances in energy-efficient technologies and the use of renewable energy sources can help reduce energy costs and improve sustainability.
- Labor and Operational Costs: Labor costs include the wages of skilled chemists, engineers, and quality control staff required to run the Amiodarone production facility. Additionally, ongoing training, safety measures, and operational efficiency are critical factors in maintaining production levels and minimizing downtime. Operational costs also include expenses related to quality control, packaging, and logistics.
- Plant Maintenance and Depreciation: Regular maintenance of equipment, such as reactors, filtration units, and crystallizers, is necessary to ensure the smooth operation of the Amiodarone production plant. The depreciation of equipment and infrastructure also needs to be accounted for in the overall cost analysis. Proper maintenance and timely upgrades can help extend the lifespan of the equipment and reduce the risk of production disruptions.
- Regulatory Compliance Costs: Compliance with GMP and other regulatory standards is essential for the production of pharmaceutical-grade Amiodarone. The costs associated with implementing and maintaining these measures, including quality control systems, audits, and certifications, must be factored into the overall production costs. Investing in regulatory compliance not only ensures product safety but also enhances marketability and access to global markets.
Looking for an Exhaustive and Personalized Report That Could Significantly Substantiate Your Business?
If you are seeking a more detailed and customized analysis of the Amiodarone production process, including cost assessments, market insights, and strategic recommendations, we can provide a comprehensive report tailored to your specific business needs. Our personalized reports offer in-depth information that can help you make informed decisions, optimize production processes, and enhance profitability. Whether you are a producer, investor, or stakeholder in the pharmaceutical industry, our reports are designed to substantiate your business with accurate and actionable data.
About Us:
Procurement Resource is an invaluable partner for businesses seeking comprehensive market research and strategic insights across a spectrum of industries. With a repository of over 500 chemicals, commodities, and utilities, updated regularly, they offer a cost-effective solution for diverse procurement needs. Their team of seasoned analysts conducts thorough research, delivering clients with up-to-date market reports, cost models, price analysis, and category insights.
By tracking prices and production costs across various goods and commodities, Procurement Resource ensures clients receive the latest and most reliable data. Collaborating with procurement teams across industries, they provide real-time facts and pioneering practices to streamline procurement processes and enable informed decision-making. Procurement Resource empowers clients to navigate complex supply chains, understand industry trends, and develop strategies for sustainable growth.
Contact Us:
Company Name: Procurement Resource
Contact Person: Amanda Williams
Email: sales@procurementresource.com
Toll-Free Number: USA Canada – Phone no: +1 307 363 1045 | UK – Phone no: +44 7537 132103 | Asia-Pacific (APAC) – Phone no: +91 1203185500
Address: 30 North Gould Street, Sheridan, WY 82801, USA